
SERVICES AND SOLUTIONS
Vantage BioTrials offers a wide range of clinical trial management services to its clients in the pharmaceutical and medical device industry by implementing Quality by Design (QbD) strategies, Risk-Based Approaches to Monitoring and process improvement methods to the entire clinical trial lifespan.
Through our unique model, we continue to perform international trials by offering expert services which result in flexibility of choosing full service or a-la-carte.
When planning your next Bioequivalence or Phase I trial, look no further than Vantage BioTrials to deliver the following range of services to accomplish your goals:
- Study Design
- Feasibility Assessments
- Protocol Development
- Site Selection
- Project Management
- Clinical Monitoring (Bilingual)
- Case Report Form Design
- Document Management
- Medical Writing
- Quality Assurance (GCP/GLP Audits)
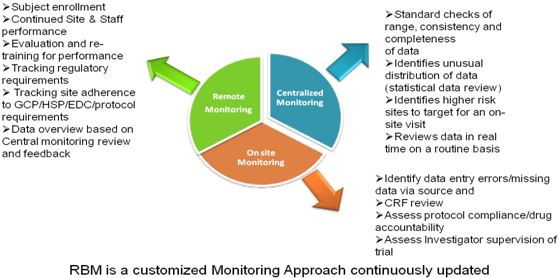
With hundreds of Bioequivalence/Phase I trials monitored to date for our Generic Pharmaceutical, Consumer Health, Biotechnology and Biopharma Sponsors, we are the only expert service provider in Canada with so much experience in handling these type of early-phase trials. With our Risk Based Approach to monitoring, we will ensure:
- Interpreting the metrics, performance indicators, and trends that emerge from centralized monitoring to ensure proactive decision making and interventions
- Ensuring continuous flow of study data that is being monitored for trends to enable real-time decision making and action
- Qualitative reports
When planning your next Bioequivalence or Phase I trial, look no further than Vantage BioTrials to deliver the following range of services to accomplish your goals:
- Study Design
- Feasibility Assessments
- Protocol Development
- Site Selection
- Project Management
- Clinical Monitoring (Bilingual)
- Case Report Form Design
- Document Management
- Medical Writing
- Quality Assurance (GCP/GLP Audits)
With hundreds of Bioequivalence/Phase I trials monitored to date for our Generic Pharmaceutical, Consumer Health, Biotechnology and Biopharma Sponsors, we are the only expert service provider in Canada with so much experience in handling these type of early-phase trials. With our Risk Based Approach to monitoring, we will ensure:
- Interpreting the metrics, performance indicators, and trends that emerge from centralized monitoring to ensure proactive decision making and interventions
- Ensuring continuous flow of study data that is being monitored for trends to enable real-time decision making and action
- Qualitative reports

With hundreds of highly-skilled clinical research consultants working for us throughout North America, in addition to more than 50 in Latin America, we bring the best talent and resources to the table for your late-phase studies. Our monitors and project managers come with more than 10 years experience in Phase II-III studies. Rest assured that we strive in assigning the best for your trial management needs. As an added value, all of our associates are specially trained to trouble-shoot, manage and deliver quality results in a very pro-active way. The following is a list of services we offer for Phase II-III studies:
- Study Design
- Feasibility Assessments
- Protocol Development
- Site Selection
- Project Management
- Clinical Monitoring (Bilingual)
- Pharmacovigilance
- Regulatory
- Vendor Management
- Data Management & EDC Services
- Biostatistical Analysis
- Investigator Meeting Planning
- Patient Recruitment Services
- Case Report Form Design
- Clinical Trial Agreements (CTAs)
- Document Management
- Medical Writing
- Quality Assurance Audits
With the increase of global Regulatory Agencies’ demand for Late Phase programs (IIIB, IV, patient registries and observational studies), these studies have become an integral role in biopharmaceutical and medical device manufacturers’ clinical, risk management, and commercialization efforts for products.
Vantage BioTrials has specific experience and expertise in the Late Phase arena to address regulatory & risk management considerations, create meaningful data reports, and provide disease/ product information from Late Stage & Post Marketing studies.
At Vantage BioTrials, our experience encompasses all Late Phase studies which include:
- Post-Marketing Safety studies
- Patient Registries
- Observational (non-intervention) studies
- Quality of Life (health and economical related studies)
- Additional indication or label expansion trials
Due to the breadth of size, scope and timelines of most late phase trials, Vantage BioTrials ensures that the studies’ logistics, recruitment & site operational requirements are well assessed and maintained. Late Phase studies’ challenges including the handling of cost and extensiveness of data based on patients’ and sites’ volumes are proactively planned and executed.
Our business model provides high quality service and strategies through:
- An experienced and dedicated Project Management and Clinical Team
- Focused Site Management
- Web based technology to meet the needs of Late Stage Studies and electronic Patient Reported Outcomes
Developing the right approach by using protocol design input and working towards a specific regulatory claim and commercial application early in the life cycle is the fastest way to market for medical devices or in vitro diagnostics.
Vantage BioTrials has a deep understanding of the challenges faced in clinical development for medical devices: intense competition, precise regulatory requirements and shortened product exclusivity and life cycle. Medical devices are not like drugs or vaccines. They even differ greatly from each other. That’s why you need a Medical Device & Diagnostics group with the broad experience of our project teams. We have the capability to meet your clinical development needs for both pilot and pivotal studies using efficient study management strategies that can reduce costs and shorten time lines by:
- Developing a well-designed protocol and case report forms (CRFs) to ensure high quality data and efficient monitoring
- Site identification and feasibility analysis
- Support Services to meet ISO 14971-2012 “Application of risk management to medical devices” requirement; management procedures and practices to analyze, evaluate, control, and monitor risk relating to the safety of a medical device throughout the protocol design, development and product lifecycle
- Application to Health Authorities, IRB/EC submission, labeling assistance, and other global requirements. Efficiently managing study start up, and execution in accordance with ICH GCP & ISO 14155-2011
- Risk analysis/mitigation and risk-based monitoring approach
As one of the only Canadian CRO’s that has established a niche for the management of Medical Device trials, Vantage BioTrials has successfully obtained numerous agency approvals, PMAs and FDA 510(k) clearances.
Our experience includes:
- Proof of Concept Studies
- Companion Diagnostics
- Implantables
- Combined Device/Drug Systems
- Orthopedics
“Quality is never an accident; it is always the result of high intention, sincere effort, intelligent direction and skillful execution.” – William A. Foster
Quality is one of Vantage BioTrials’ most important elements when conducting monitoring or quality assurance audits of investigational sites or Contract Research Organizations.
Here’s a list of what we can do for you to help maintain Quality for your studies:
- Generate audit reports and review for accuracy, clarity and completeness.
- Ensure that impact/validity assessments are clearly written and, where appropriate, support the facts.
- Manage event investigation process and ensure regulatory compliance for all studies reviewed.
- Conduct general inspections/monitoring of Phase I-IV studies in compliance to Protocols, SOPs, GLPs, GCPs and generally accepted scientific principles.
- Audit raw data records for completeness and within compliance to Protocols, SOPs, GLPs, GCPs, 21 CFR Part 11 and generally accepted scientific principles.
- Audit of reports to ensure that the results incorporated accurately reflect the raw data.
- Gap analysis of processes and organization
- Vendor audits
- Quality System Optimization including development of quality manuals and policies, SOPs, work instructions, QA processes, training, and regulatory inspection readiness programs.
Site/Study Rescue:
Unfortunately it is a fact of life in clinical research to encounter issues that negatively affect or hinder the progress of your studies, especially with problematic investigative sites or poor quality of work provided by other CROs. With our experience handling complicated trials, our Sponsors come to us to implement our Strategic Working Action Teams (SWAT) into a cohesive unit to address the issues and efficiently rescue your study from disastrous consequences. Our SWAT team is comprised of a small number of carefully chosen experts (including at least one senior project manager and clinical monitor) who come up with action plans to get your study back on the right track…


